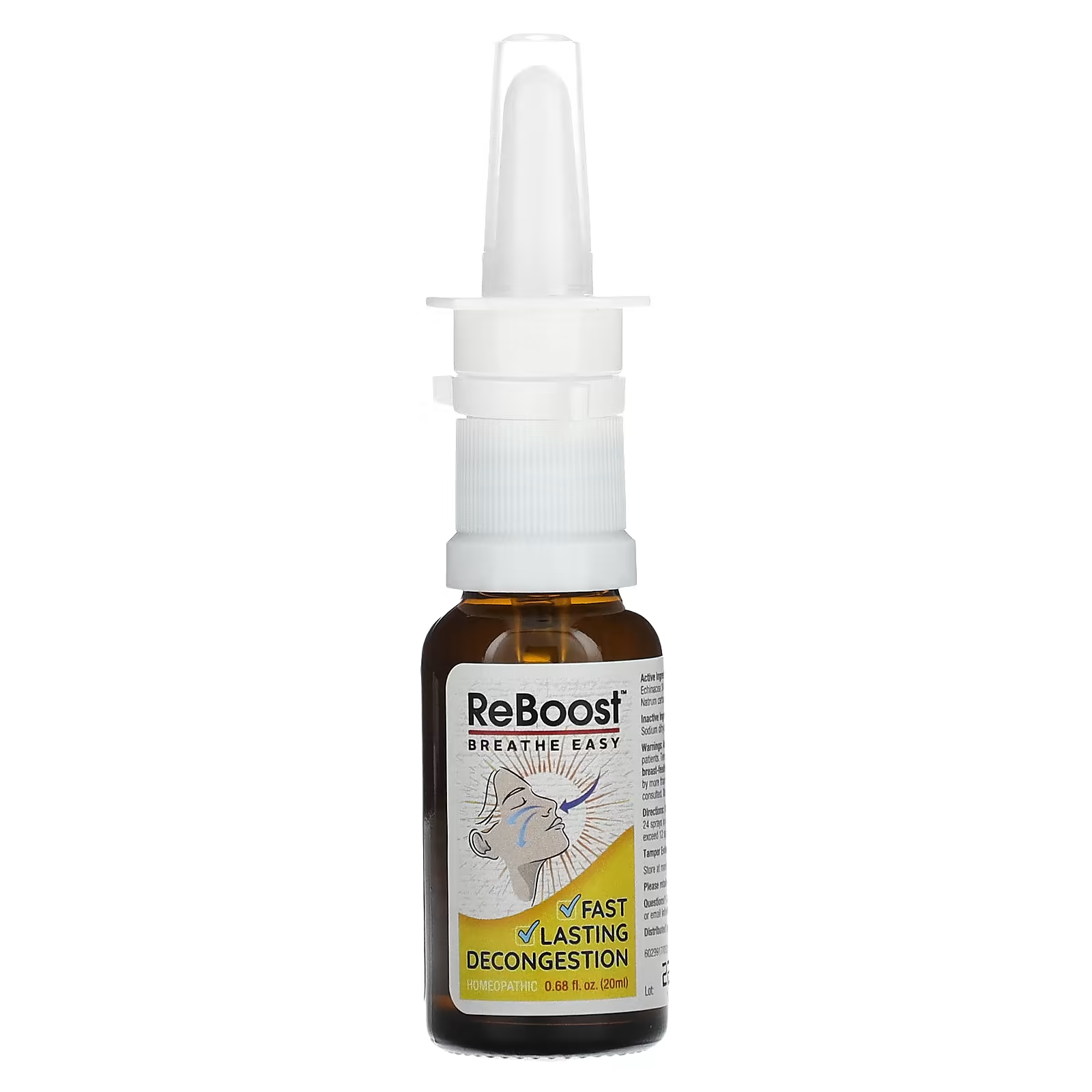
ReBoost Nasal Spray has come under national attention after health officials confirmed mold and bacterial contamination in certain batches. The issue has triggered a nationwide recall and raised serious concerns about consumer safety, especially for people with weak immune systems. Authorities are advising users to stop using the product immediately and check their packaging details carefully.
This development has left many consumers questioning how a commonly used nasal spray could pose such serious health risks.
What Triggered the Nationwide Recall
The recall was announced after testing revealed the presence of yeast, mold, and microbial contamination in ReBoost Nasal Spray. One of the bacteria identified during testing was Achromobacter, which was found at levels higher than safety standards allow.
Health officials warned that there is a real possibility of harmful health effects from using the contaminated spray. In certain cases, especially among immunocompromised individuals, these effects could become life-threatening. This warning has pushed the product under close scrutiny by regulators and public health agencies.
Health Risks and Symptoms to Know
Using a contaminated nasal spray can introduce harmful microorganisms directly into the nasal passages. Reported symptoms that may be linked to this exposure include fever, sinus swelling, headaches, facial pain or pressure, and numbness in the face.
People experiencing any of these symptoms after using ReBoost Nasal Spray are encouraged to contact a healthcare provider promptly. You can also review our internal article on early signs of infection to better understand when medical attention is needed.
How to Identify the Affected Product
Not all ReBoost Nasal Spray products are impacted. Consumers should carefully check the details on their packaging.
| Product Detail | Information |
|---|---|
| Product Name | ReBoost Nasal Spray |
| Lot Number | 224268 |
| Expiration Date | December 2027 |
| Bottle Size | 20 mL |
| Packaging | White and yellow carton |
If your product matches these details, discontinue use right away.
What Consumers Should Do Next
Customers who purchased the nasal spray directly from the manufacturer can request a refund through the company’s recall process. Those who bought it from stores or online marketplaces should return it to the original place of purchase.
So far, there have been no confirmed reports of serious adverse events. However, health officials stress that stopping use early is key to preventing potential complications. For more safety tips, see our internal guide on proper nasal spray use and storage.
Why This Recall Matters
ReBoost Nasal Spray is a homeopathic product made with natural ingredients like echinacea and is marketed for nasal congestion relief. While many people associate natural products with safety, this recall highlights that contamination risks can exist in any health product if quality controls fail.
This situation serves as a reminder for consumers to stay alert to recall notices and act quickly when warnings are issued. Being informed helps reduce unnecessary health risks and ensures safer everyday choices for you and your family.